anti-Hsp 90 antibody [H90-10]
| 产品描述 | Mouse Monoclonal antibody [H90-10] recognizes Hsp 90 |
|---|---|
| 反应物种 | Hu, Ms, Rat, Chk, Dog, Fsh, Rb, Shark |
| 应用 | ELISA, ICC/IF, IHC, IP, WB |
| 特异性 | Human (beta-specific), Rabbit (Beta Specific), Chicken (Alpha/Beta), Rat, Canine, Catostomus commersonii (fish) |
| 宿主 | Mouse |
| 克隆 | Monoclonal |
| 克隆号 | H90-10 |
| 同位型 | IgG2a |
| 靶点名称 | Hsp 90 |
| 抗原物种 | Human |
| 抗原 | Recombinant Human Hsp90 beta (NP_031381.2). |
| 偶联标记 | Un-conjugated |
| 別名 | HSPC2; D6S182; Heat shock 84 kDa; HSP90B; HSP84; HSP 84; Heat shock protein HSP 90-beta; HSP 90; HSPCB |
| 交叉反应性说明 | Human (beta-specific), Rabbit (Beta Specific), Chicken (Alpha/Beta), Rat, Canine, Catostomus commersonii (fish) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 应用建议 |
| ||||||||||||
| 应用说明 | * The dilutions indicate recommended starting dilutions and the optimal dilutions or concentrations should be determined by the scientist. |
| 形式 | Liquid |
|---|---|
| 纯化 | Purification with Protein G. |
| 缓冲液 | PBS (pH 7.4), 0.09% Sodium azide and 50% Glycerol |
| 抗菌剂 | 0.09% Sodium azide |
| 稳定剂 | 50% Glycerol |
| 浓度 | 1 mg/ml |
| 存放说明 | For continuous use, store undiluted antibody at 2-8°C for up to a week. For long-term storage, aliquot and store at -20°C. Storage in frost free freezers is not recommended. Avoid repeated freeze/thaw cycles. Suggest spin the vial prior to opening. The antibody solution should be gently mixed before use. |
| 注意事项 | For laboratory research only, not for drug, diagnostic or other use. |
| 数据库连接 | |
|---|---|
| 基因名称 | HSP90AB1 |
| 全名 | heat shock protein 90kDa alpha (cytosolic), class B member 1 |
| 背景介绍 | HSP90 is an abundantly and ubiquitously expressed heat shock protein. It is understood to exist in two principal forms α and β, which share 85% sequence amino acid homology. The two isoforms of Hsp90, are expressed in the cytosolic compartment (1). Despite the similarities, HSP90α exists predominantly as a homodimer while HSP90β exists mainly as a monomer.(2) From a functional perspective, hsp90 participates in the folding, assembly, maturation, and stabilization of specific proteins as an integral component of a chaperone complex. (3-6) Furthermore, Hsp90 is highly conserved between species; having 60% and 78% amino acid similarity between mammalian and the corresponding yeast and Drosophila proteins, respectively. Hsp90 is a highly conserved and essential stress protein that is expressed in all eukaryotic cells. Despite it’s label of being a heat-shock protein, hsp90 is one of the most highly expressed proteins in unstressed cells (1–2% of cytosolic protein). It carries out a number of housekeeping functions – including controlling the activity, turnover, and trafficking of a variety of proteins. Most of the hsp90-regulated proteins that have been discovered to date are involved in cell signaling. (7-8). The number of proteins now know to interact with Hsp90 is about 100. Target proteins include the kinases v-Src, Wee1, and c-Raf, transcriptional regulators such as p53 and steroid receptors, and the polymerases of the hepatitis B virus and telomerase.5 When bound to ATP, Hsp90 interacts with co-chaperones Cdc37, p23, and an assortment of immunophilin-like proteins, forming a complex that stabilizes and protects target proteins from proteasomal degradation. In most cases, hsp90-interacting proteins have been shown to co-precipitate with hsp90 when carrying out immunoadsorption studies, and to exist in cytosolic heterocomplexes with it. In a number of cases, variations in hsp90 expression or hsp90 mutation has been shown to degrade signaling function via the protein or to impair a specific function of the protein (such as steroid binding, kinase activity) in vivo. Ansamycin antibiotics, such as geldanamycin and radicicol, inhibit hsp90 function (9). 1. Nemoto T., et al. (1997) J.Biol Chem. 272: 26179-26187. 2. Minami Y., et al. (1991), J.Biol Chem. 266: 10099-10103. 3. Arlander S.J.H., et al. (2003) J Biol Chem 278: 52572-52577. 4. Pearl H., et al. (2001) Adv Protein Chem 59:157-186. 5. Neckers L., et al. (2002) Trends Mol Med 8:S55-S61. 6. Pratt W., Toft D. (2003) Exp Biol Med 228:111-133. 7. Pratt W., Toft D. (1997) Endocr Rev 18:306–360. 8. Pratt W.B. (1998) Proc Soc Exptl Biol Med 217: 420–434. 9. Whitesell L., et al. (1994) Proc Natl Acad Sci USA 91: 8324–8328. |
| 生物功能 | Molecular chaperone that promotes the maturation, structural maintenance and proper regulation of specific target proteins involved for instance in cell cycle control and signal transduction. Undergoes a functional cycle that is linked to its ATPase activity. This cycle probably induces conformational changes in the client proteins, thereby causing their activation. Interacts dynamically with various co-chaperones that modulate its substrate recognition, ATPase cycle and chaperone function. [UniProt] |
| 研究领域 | Cancer antibody; Signaling Transduction antibody |
| 预测分子量 | 83 kDa |
| 翻译后修饰 | Ubiquitinated in the presence of STUB1-UBE2D1 complex (in vitro). ISGylated. S-nitrosylated; negatively regulates the ATPase activity. Phosphorylation at Tyr-301 by SRC is induced by lipopolysaccharide (PubMed:23585225). Phosphorylation at Ser-226 and Ser-255 inhibits AHR interaction (PubMed:15581363). Methylated by SMYD2; facilitates dimerization and chaperone complex formation; promotes cancer cell proliferation. Cleaved following oxidative stress resulting in HSP90AB1 protein radicals formation; disrupts the chaperoning function and the degradation of its client proteins. |
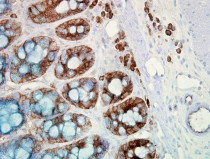
ARG20503 anti-Hsp 90 antibody [H90-10] IHC image
Immunohistochemistry: cancerous Human colon tissue stained with ARG20503 anti-Hsp 90 antibody [H90-10].
ARG20503 anti-Hsp 90 antibody [H90-10] WB image
Western blot: cell lysates from 12 Human cancer cell lines stained with ARG20503 anti-Hsp 90 antibody [H90-10] at 1:1000 dilution.
ARG20503 anti-Hsp 90 antibody [H90-10] IHC image
Immunohistochemistry: cancerous Human colon tissue stained with ARG20503 anti-Hsp 90 antibody [H90-10].
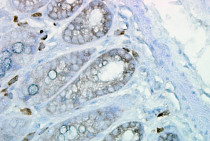
ARG20503 anti-Hsp 90 antibody [H90-10] IHC image
Immunohistochemistry: cancerous Mouse colon tissue stained with ARG20503 anti-Hsp 90 antibody [H90-10].
ARG20503 anti-Hsp 90 antibody [H90-10] IHC image
Immunohistochemistry: cancerous Mouse colon tissue stained with ARG20503 anti-Hsp 90 antibody [H90-10].
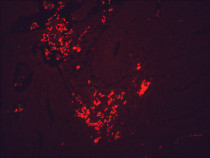
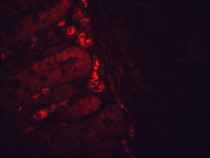
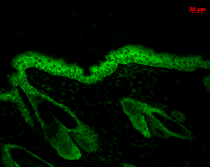
ARG20503 anti-Hsp 90 antibody [H90-10] IHC-P image
Immunohistochemistry: Bouin's fixed and paraffin-embedded Mouse backskin stained with ARG20503 anti-Hsp 90 antibody [H90-10] at 1:100 dilution (1 hour, RT). Secondary antibody: FITC Goat anti-Mouse (green) at 1:50 dilution (1 hour at RT). Localization: Epidermis.
ARG20503 anti-Hsp 90 antibody [H90-10] WB image
Western blot: HeLa cell lysates stained with ARG20503 anti-Hsp 90 antibody [H90-10] at 1:1000 dilution.
克隆号文献
 New Products
New Products




![anti-Hsp 90 antibody [H90-10]](/upload/image/products/ARG20503_7_210_205.jpg)
![anti-Hsp 90 antibody [H90-10]](/upload/image/products/ARG20503_1.jpg)
![anti-Hsp 90 antibody [H90-10]](/upload/image/products/ARG20503_5.jpg)
![anti-Hsp 90 antibody [H90-10]](/upload/image/products/ARG20503_2.jpg)
![anti-Hsp 90 antibody [H90-10]](/upload/image/products/ARG20503_3.jpg)
![anti-Hsp 90 antibody [H90-10]](/upload/image/products/ARG20503_4.jpg)
![anti-Hsp 90 antibody [H90-10]](/upload/image/products/ARG20503_IHC_Mouse_backskin.jpg)
![anti-Hsp 90 antibody [H90-10]](/upload/image/products/ARG20503_7.jpg)