anti-PML antibody
CAT.NO. : ARG66699
US$ Please choose
US$ Please choose
Size:
Trail, Bulk size or Custom requests Please contact us
概述
| 产品描述 | Rabbit Polyclonal antibody recognizes PML |
|---|---|
| 反应物种 | Hu, AGMK |
| 应用 | ICC/IF, IHC-P, WB |
| 宿主 | Rabbit |
| 克隆 | Polyclonal |
| 同位型 | IgG |
| 靶点名称 | PML |
| 抗原物种 | Human |
| 抗原 | Synthetic peptide within aa. 30-110 of Human PML. |
| 偶联标记 | Un-conjugated |
| 別名 | RING finger protein 71; RNF71; Promyelocytic leukemia protein; TRIM19; Protein PML; PP8675; MYL; Tripartite motif-containing protein 19 |
应用说明
| 应用建议 |
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 应用说明 | * The dilutions indicate recommended starting dilutions and the optimal dilutions or concentrations should be determined by the scientist. | ||||||||
| 实际分子量 | ~ 90 kDa |
属性
| 形式 | Liquid |
|---|---|
| 纯化 | Affinity purification with immunogen. |
| 缓冲液 | PBS, 0.02% Sodium azide, 50% Glycerol and 0.5% BSA. |
| 抗菌剂 | 0.02% Sodium azide |
| 稳定剂 | 50% Glycerol and 0.5% BSA |
| 浓度 | 1 mg/ml |
| 存放说明 | For continuous use, store undiluted antibody at 2-8°C for up to a week. For long-term storage, aliquot and store at -20°C. Storage in frost free freezers is not recommended. Avoid repeated freeze/thaw cycles. Suggest spin the vial prior to opening. The antibody solution should be gently mixed before use. |
| 注意事项 | For laboratory research only, not for drug, diagnostic or other use. |
生物信息
| 数据库连接 | |
|---|---|
| 基因名称 | PML |
| 全名 | promyelocytic leukemia |
| 背景介绍 | The protein encoded by this gene is a member of the tripartite motif (TRIM) family. The TRIM motif includes three zinc-binding domains, a RING, a B-box type 1 and a B-box type 2, and a coiled-coil region. This phosphoprotein localizes to nuclear bodies where it functions as a transcription factor and tumor suppressor. Its expression is cell-cycle related and it regulates the p53 response to oncogenic signals. The gene is often involved in the translocation with the retinoic acid receptor alpha gene associated with acute promyelocytic leukemia (APL). Extensive alternative splicing of this gene results in several variations of the protein's central and C-terminal regions; all variants encode the same N-terminus. Alternatively spliced transcript variants encoding different isoforms have been identified. [provided by RefSeq, Jul 2008] |
| 生物功能 | Functions via its association with PML-nuclear bodies (PML-NBs) in a wide range of important cellular processes, including tumor suppression, transcriptional regulation, apoptosis, senescence, DNA damage response, and viral defense mechanisms. Acts as the scaffold of PML-NBs allowing other proteins to shuttle in and out, a process which is regulated by SUMO-mediated modifications and interactions. Isoform PML-4 has a multifaceted role in the regulation of apoptosis and growth suppression: activates RB1 and inhibits AKT1 via interactions with PP1 and PP2A phosphatases respectively, negatively affects the PI3K pathway by inhibiting MTOR and activating PTEN, and positively regulates p53/TP53 by acting at different levels (by promoting its acetylation and phosphorylation and by inhibiting its MDM2-dependent degradation). Isoform PML-4 also: acts as a transcriptional repressor of TBX2 during cellular senescence and the repression is dependent on a functional RBL2/E2F4 repressor complex, regulates double-strand break repair in gamma-irradiation-induced DNA damage responses via its interaction with WRN, acts as a negative regulator of telomerase by interacting with TERT, and regulates PER2 nuclear localization and circadian function. Isoform PML-6 inhibits specifically the activity of the tetrameric form of PKM. The nuclear isoforms (isoform PML-1, isoform PML-2, isoform PML-3, isoform PML-4 and isoform PML-5) in concert with SATB1 are involved in local chromatin-loop remodeling and gene expression regulation at the MHC-I locus. Isoform PML-2 is required for efficient IFN-gamma induced MHC II gene transcription via regulation of CIITA. Cytoplasmic PML is involved in the regulation of the TGF-beta signaling pathway. PML also regulates transcription activity of ELF4 and can act as an important mediator for TNF-alpha- and IFN-alpha-mediated inhibition of endothelial cell network formation and migration. Exhibits antiviral activity against both DNA and RNA viruses. The antiviral activity can involve one or several isoform(s) and can be enhanced by the permanent PML-NB-associated protein DAXX or by the recruitment of p53/TP53 within these structures. Isoform PML-4 restricts varicella zoster virus (VZV) via sequestration of virion capsids in PML-NBs thereby preventing their nuclear egress and inhibiting formation of infectious virus particles. The sumoylated isoform PML-4 restricts rabies virus by inhibiting viral mRNA and protein synthesis. The cytoplasmic isoform PML-14 can restrict herpes simplex virus-1 (HHV-1) replication by sequestering the viral E3 ubiquitin-protein ligase ICP0 in the cytoplasm. Isoform PML-6 shows restriction activity towards human cytomegalovirus (HCMV) and influenza A virus strains PR8(H1N1) and ST364(H3N2). Sumoylated isoform PML-4 and isoform PML-12 show antiviral activity against encephalomyocarditis virus (EMCV) by promoting nuclear sequestration of viral polymerase (P3D-POL) within PML NBs. Isoform PML-3 exhibits antiviral activity against poliovirus by inducing apoptosis in infected cells through the recruitment and the activation of p53/TP53 in the PML-NBs. Isoform PML-3 represses human foamy virus (HFV) transcription by complexing the HFV transactivator, bel1/tas, preventing its binding to viral DNA. PML may positively regulate infectious hepatitis C viral (HCV) production and isoform PML-2 may enhance adenovirus transcription. [UniProt] |
| 细胞定位 | Nucleus, Endosome and Endoplasmic reticulum. [UniProt] |
| 预测分子量 | 98 kDa |
| 翻译后修饰 | Ubiquitinated; mediated by RNF4, RNF111, UHRF1, UBE3A/E6AP, BCR(KLHL20) E3 ubiquitin ligase complex E3 ligase complex, SIAH1 or SIAH2 and leading to subsequent proteasomal degradation (PubMed:18408734, PubMed:21840486, PubMed:22033920). Ubiquitination by BCR(KLHL20) E3 ubiquitin ligase complex E3 ligase complex requires CDK1/2-mediated phosphorylation at Ser-518 which in turn is recognized by prolyl-isopeptidase PIN1 and PIN1-catalyzed isomerization further potentiates PML interaction with KLHL20 (PubMed:21840486, PubMed:22033920). 'Lys-6'-, 'Lys-11'-, 'Lys-48'- and 'Lys-63'-linked polyubiquitination by RNF4 is polysumoylation-dependent (PubMed:18408734). Ubiquitination by RNF111 is polysumoylation-dependent (By similarity). Sumoylation regulates PML's: stability in response to extracellular or intracellular stimuli, transcription directly and indirectly, through sequestration of or dissociation of the transcription factors from PML-NBs, ability to regulate apoptosis and its anti-viral activities. It is also essential for: maintaining proper PML nuclear bodies (PML-NBs) structure and normal function, recruitment of components of PML-NBs, the turnover and retention of PML in PML-NBs and the integrity of PML-NBs. Undergoes 'Lys-11'-linked sumoylation. Sumoylation on all three sites (Lys-65, Lys-160 and Lys-490) is required for nuclear body formation. Sumoylation on Lys-160 is a prerequisite for sumoylation on Lys-65. Lys-65 and Lys-160 are sumoylated by PISA1 and PIAS2. PIAS1-mediated sumoylation of PML promotes its interaction with CSNK2A1/CK2 and phosphorylation at Ser-565 which in turn triggers its ubiquitin-mediated degradation. PIAS1-mediated sumoylation of PML-RARA promotes its ubiquitin-mediated degradation. The PML-RARA fusion protein requires the coiled-coil domain for sumoylation. Sumoylation at Lys-490 by RANBP2 is essential for the proper assembly of PML-NBs. DNA damage triggers its sumoylation while some but not all viral infections can abolish sumoylation. Desumoylated by SENP1, SENP2, SENP3, SENP5 and SENP6. Arsenic induces PML and PML-RARA polysumoylation and their subsequent RNF4-dependent ubiquitination and proteasomal degradation, and is used as treatment in acute promyelocytic leukemia (APL). The nuclear isoforms (isoform PML-1, isoform PML-2, isoform PML-3, isoform PML-4, isoform PML-5 and isoform PML-6) show an increased sumoylation in response to arsenic trioxide. The cytoplasmic isoform PML-7 is not sumoylated. Phosphorylation is a major regulatory mechanism that controls PML protein abundance and the number and size of PML nuclear bodies (PML-NBs). Phosphorylated in response to DNA damage, probably by ATR. HIPK2-mediated phosphorylation at Ser-8, Ser-36 and Ser-38 leads to increased accumulation of PML protein and its sumoylation and is required for the maximal pro-apoptotic activity of PML after DNA damage. CHEK2-mediated phosphorylation at Ser-117 is important for PML-mediated apopotosis following DNA damage. MAPK1-mediated phosphorylations at Ser-403, Ser-505, Ser-527 and Ser-530 and CDK1/2-mediated phosphorylation at Ser-518 promote PIN1-dependent PML degradation. CK2-mediated phosphorylation at Ser-565 primes PML ubiquitination via an unidentified ubiquitin ligase. Acetylation at Lys-487 is essential for its nuclear localization. Deacetylated at Lys-487 by SIRT1 and this deacetylation promotes PML control of PER2 nuclear localization. [UniProt] |
检测图片 (4)
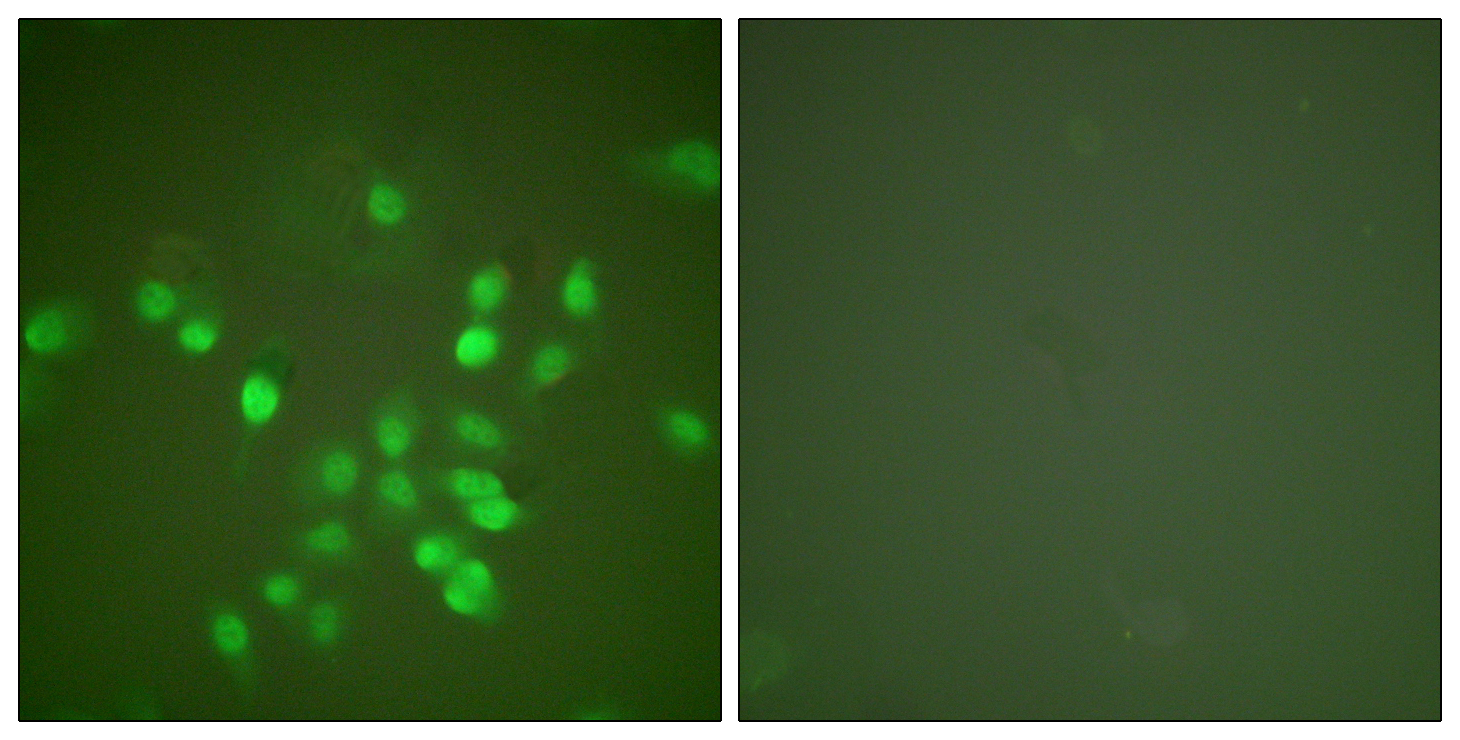
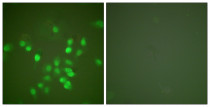
ARG66699 anti-PML antibody ICC/IF image
Immunofluorescence: A549 cells stained with ARG66699 anti-PML antibody. The picture on the right is blocked with the synthetic peptide.
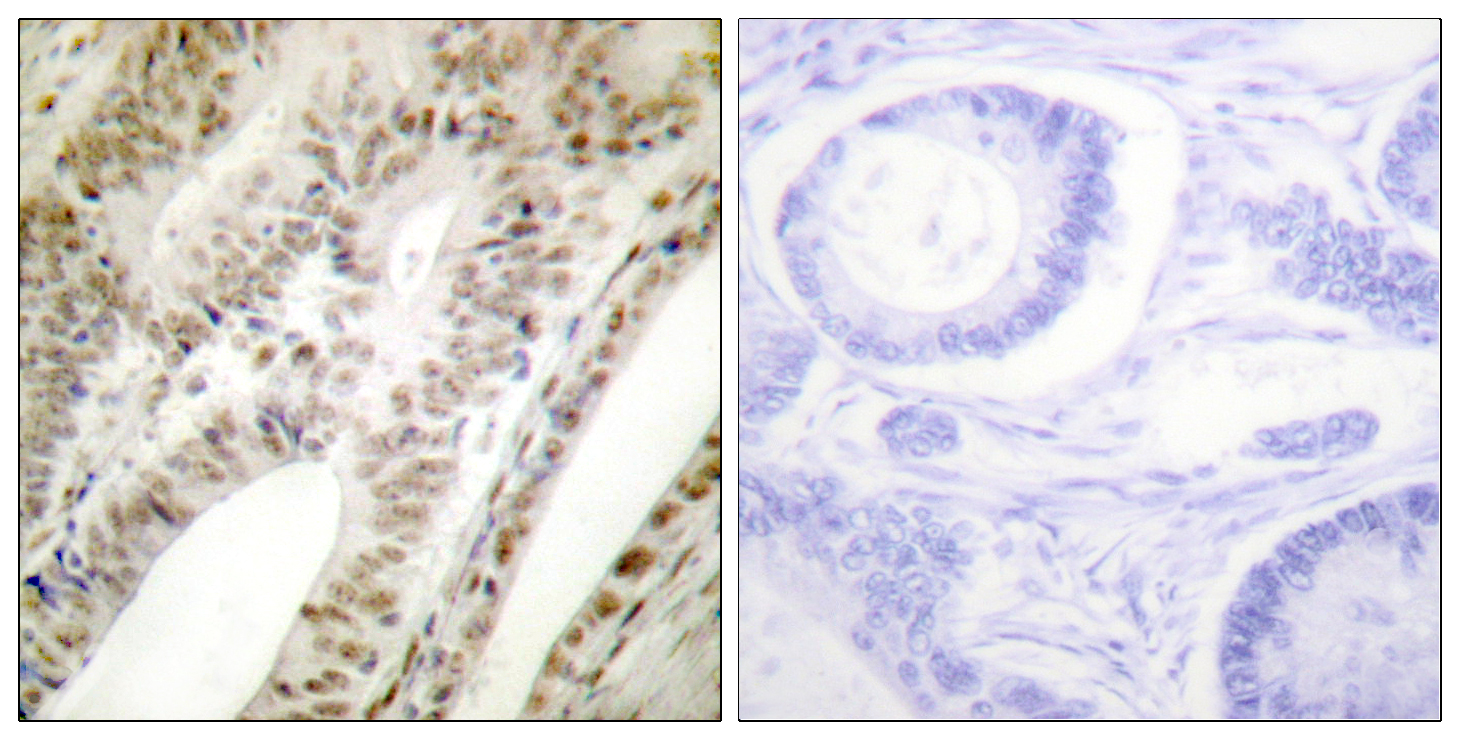
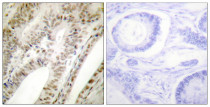
ARG66699 anti-PML antibody IHC-P image
Immunohistochemistry: Paraffin-embedded Human tonsil tissue stained with ARG66699 anti-PML antibody. The picture on the right is blocked with the synthetic peptide.
ARG66699 anti-PML antibody WB image
Western blot: A549 cell lysate stained with ARG66699 anti-PML antibody at 1:1000 dilution.
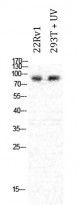
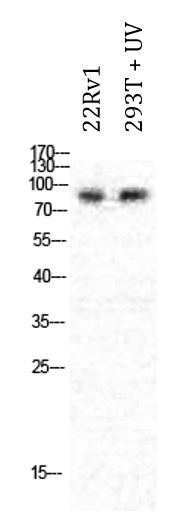
ARG66699 anti-PML antibody WB image
Western blot: 22Rv1 and 293T treated with UV. Cell lysates were stained with ARG66699 anti-PML antibody at 1:1000 dilution.
 New Products
New Products